Comparing the COVID-19 Tests
Which is the BEST TEST for your Program?
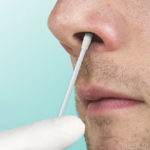
Viral Antigen Test
Specificity (Positive): 100%
Sensitivity (Negative): 97%
Test Type: Front of nose swab
Best Results for Infected Person: Anytime after Day 2 of infection
Set up Time: 16 minutes
Result Time: 11 seconds
Same Day Results? YES
Send to Lab? NO
Discomfort Level: NONE
Average Cost: $40
This test is now the MOST ACCURATE TEST on the Market today. A Viral Antigen is a molecule present on the outside of a virus. Antigen tests are used for early identification of an active infection. Viral antigens can be detected in samples taken from the nasal passages or the throat. Antibodies located on the COVID-19 test kit bind with the viral antigen from the sample if the individual is infected with COVID-19
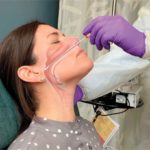
PCR Test
Specificity (Positive): 100%
Sensitivity (Negative): 95%
Test Type: Back of Nasal Passage (Nasopharynx)
Best Results for Infected Person: Between Day 3 and Day 7 of infection
Set up Time: 5 minutes
Result Time: 1-7 Days*
Same Day Results? NO
Send to Lab? YES
Discomfort Level: HIGH
Average Cost: $110
Sometimes called “molecular photocopying,” the polymerase chain reaction (PCR) is labor intensive and expensive technique used to “amplify” – copy – small segments of DNA. Because significant amounts of a sample of DNA are necessary for molecular and genetic analyses, studies of isolated pieces of DNA are nearly impossible without PCR amplification.
*Labs are currently backed up, leading to longer wait times for results
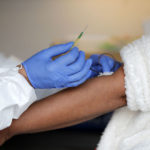
Antibody Test
Specificity (Positive): Unknown
Sensitivity (Negative): Unknown
Test Type: Blood Draw
Best Results for Infected Person: Unknown
Set up Time: 5 minutes
Result Time: 1-5 Days*
Same Day Results? NO
Send to Lab? YES
Discomfort Level: MEDIUM
Average Cost: $100
Antibody results may help identify if you were exposed to the virus that causes COVID-19 and, if so, whether or not your body has developed antibodies. Although having antibodies usually gives immunity from further infection, there is not enough evidence at this time to suggest that people who have antibodies against SARS-CoV-2, the virus that causes COVID-19, are protected against future infections from the virus. Results from this test also will not provide information on whether you can spread the virus to others and is not used as a basis for diagnosis.
*Labs are currently backed up, leading to longer wait times for results
"NAIA now required Athletes to have a PCR test performed 5 days prior to competition. Does the SOFIA2 qualify?"
PCR Testing is Viral Antigen testing. The difference is how you obtain the results. PCR test uses a lab and human reading. The Sofia test, has the Antigen on the cassette, thus allowing the computer to read if the virus binds to the antigen or not. This allows for a RAPID response. The accuracy of the Sofia test is higher than the PCR test. This is why every major professional sports organization is using the Sofia-2 to test their athletes.

Dr. James Pickney II, M.D.
Diamond Physicians
“PCR and Sofia 2 SARS testing both look at antigens. PCR amplifies the DNA/RNA first which is why the sensitivity is higher. 2-3 antigens can be made into thousands. However the process of collection and running the test is difficult. The Sofia 2 SARS antigen cassette has COVID19 antibodies on it. The antigens (from the nasal swab) go through the cassette, bond to the line on the cassette if they are present. PCR tests are used to directly detect the presence of an antigen, rather than the presence of the body’s immune response, or antibodies. By detecting viral RNA, which will be present in the body before antibodies form or symptoms of the disease are present, the tests can tell whether or not someone has the virus very early on. It’s worth noting that PCR tests can be very labour intensive, with several stages at which errors may occur between sampling and analysis. False negatives can occur up to 30% of the time with different PCR tests, meaning they’re more useful for confirming the presence of an infection than giving a patient the all-clear”

This Fact Sheet informs you of the significant known and potential risks and benefits of the emergency use of the Lyra SARS-CoV-2 Assay. The Lyra SARS-CoV-2 Assay is authorized for use on respiratory specimens from individuals suspected of COVID-19 by their healthcare provider.
All patients whose specimens are tested with this assay will receive the Fact Sheet for Patients: Lyra SARS-CoV-2 Assay
This test is to be performed only using respiratory specimens collected from individuals suspected of COVID-19 by their healthcare provider
“What are the symptoms of COVID-19?”
Many patients with confirmed COVID-19 have developed fever and/or symptoms of acute respiratory illness (e.g., cough, difficulty breathing). However, limited information is currently available to characterize the full spectrum of clinical illness associated with COVID-19. Based on what is known about the virus that causes COVID-19, signs and symptoms may appear any time from 2 to 14 days after exposure to the virus. Based on preliminary data, the median incubation period is approximately 5 days, but may range 2-14 days.
Public health officials have identified cases of COVID-19 infection throughout the world, including the United States, which may pose risks for public health. Please check the CDC webpage for the most up to date information.
“What do I need to know about COVID-19 testing?”
Current information on COVID-19 for healthcare providers is available at CDC’s webpage, Information for Healthcare Professionals (see links provided in “Where can I go for updates and more information” section).
- The Lyra SARS-CoV-2 Assay can be used to test nasal, nasopharyngeal (NP), or oropharyngeal (OP) swab
- The Lyra SARS-CoV-2 Assay should be ordered for the detection of COVID-19 in individuals suspected of COVID-19 by their healthcare
- The Lyra SARS-CoV-2 Assay is only authorized for use in laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, to perform high complexity tests.
Specimens should be collected with appropriate infection control precautions. Current guidance for COVID-19 infection control precautions are available at the CDC’s website (see links provided in “Where can I go for updates and more information” section).
Use appropriate personal protective equipment when collecting and handling specimens from individuals suspected of having COVID-19 as outlined in the CDC Interim Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated with Coronavirus Disease 2019 (COVID-19). For additional information, refer to CDC Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons Under Investigation (PUIs) for Coronavirus Disease 2019 (COVID-19) (see links provided in “Where can I go for updates and more information” section).
“What does it mean if the specimen tests positive for the virus that causes COVID-19?”
A positive test result for COVID-19 indicates that RNA from SARS-CoV-2 was detected, and the patient is infected with the virus and presumed to be contagious. Laboratory test results should always be considered in the context of clinical observations and epidemiological data in making a final diagnosis and patient management decisions. Patient management should follow current CDC guidelines.
The Lyra SARS-CoV-2 Assay has been designed to minimize the likelihood of false positive test results. However, in the event of a false positive result, risks to patients could include the following: a recommendation for isolation of the patient, monitoring of the household or other close contacts for symptoms, patient isolation that might limit contact with family or friends and may increase contact with other potentially COVID-19 patients, limits in the ability to work, the delayed diagnosis and treatment for the true infection causing the symptoms, unnecessary prescription of a treatment or therapy, or other unintended adverse effects. All laboratories using this test must follow the standard testing and reporting guidelines according to their appropriate public health authorities.
“What does it mean if the specimen tests negative for the virus that causes COVID-19?”
A negative test result for this test means that SARS- CoV-2 RNA was not present in the specimen above the limit of detection. However, a negative result does not rule out COVID-19 and should not be used as the sole basis for treatment or patient management decisions. A negative result does not exclude the possibility of COVID-19.
When diagnostic testing is negative, the possibility of a false negative result should be considered in the context of a patient’s recent exposures and the presence of clinical signs and symptoms consistent with COVID-19. The possibility of a false negative result should especially be considered if the patient’s recent exposures or clinical presentation indicate that COVID- 19 is likely, and diagnostic tests for other causes of illness (e.g., other respiratory illness) are negative. If COVID-19 is still suspected based on exposure history together with other clinical findings, re-testing should be considered by healthcare providers in consultation with public health authorities.
Risks to a patient of a false negative include: delayed or lack of supportive treatment, lack of monitoring of infected individuals and their household or other close contacts for symptoms resulting in increased risk of spread of COVID-19 within the community, or other unintended adverse events.
“What is an EUA?”
The United States FDA has made this test available under an emergency access mechanism called an Emergency Use Authorization (EUA). The EUA is supported by the Secretary of Health and Human Service’s (HHS’s) declaration that circumstances exist to justify the emergency use of in vitro diagnostics (IVDs) for the detection and/or diagnosis of the virus that causes COVID-19.
An IVD made available under an EUA has not undergone the same type of review as an FDA-approved or cleared IVD. FDA may issue an EUA when certain criteria are met, which includes that there are no adequate, approved, available alternatives, and based on the totality of scientific evidence available, it is reasonable to believe that this IVD may be effective in the detection of the virus that causes COVID-19.
The EUA for this test is in effect for the duration of the COVID-19 declaration justifying emergency use of IVDs, unless terminated or revoked (after which the test may no longer be used).
“Where can I go for updates and more information?”
CDC webpage:
General: https://www.cdc.gov/COVID19
FDA webpages:
General: www.fda.gov/novelcoronavirus
EUAs:(includes links to patient fact sheet and manufacturer’s instructions) https://www.fda.gov/medical-devices/emergency- situations-medical-devices/emergency-use-authorizations
Quidel Corporation: 9985 Summers Ridge Rd, San Diego, CA 92121 USA QDL.COV2.test.event.report@quidel.com Website: www.quidel.com
Report Adverse events, including problems with test performance or results, to MedWatch by submitting the online FDA Form 3500 (https://www.accessdata.fda.gov/scripts/medwatch/index.cfm?action=reporting.home) or by calling 1-800-FDA-1088
